# Ambient Pressure Definition and Its Importance in Science
## What Is Ambient Pressure?
Ambient pressure refers to the pressure of the surrounding environment at a given location. It is the force exerted by the atmosphere or any other surrounding medium on objects within it. Typically measured in units like pascals (Pa), atmospheres (atm), or pounds per square inch (psi), ambient pressure varies depending on altitude, weather conditions, and whether the environment is enclosed or open.
In everyday terms, we experience ambient pressure as atmospheric pressure when we’re at sea level – approximately 101,325 Pa or 1 atm. This pressure decreases as we ascend to higher altitudes where the atmosphere becomes thinner.
## How Ambient Pressure Is Measured
Scientists and engineers use various instruments to measure ambient pressure:
– Barometers: Measure atmospheric pressure
– Pressure transducers: Convert pressure into electrical signals
– Manometers: Compare pressures using liquid columns
These measurements are crucial for weather forecasting, aviation, and numerous industrial processes where pressure conditions directly affect outcomes.
## The Scientific Importance of Ambient Pressure
Understanding ambient pressure is fundamental across multiple scientific disciplines:
### 1. Physics and Chemistry
Pressure significantly influences physical and chemical processes. The ideal gas law (PV=nRT) demonstrates how pressure (P), volume (V), and temperature (T) are interrelated in gaseous systems. Many chemical reaction rates and equilibrium positions are pressure-dependent.
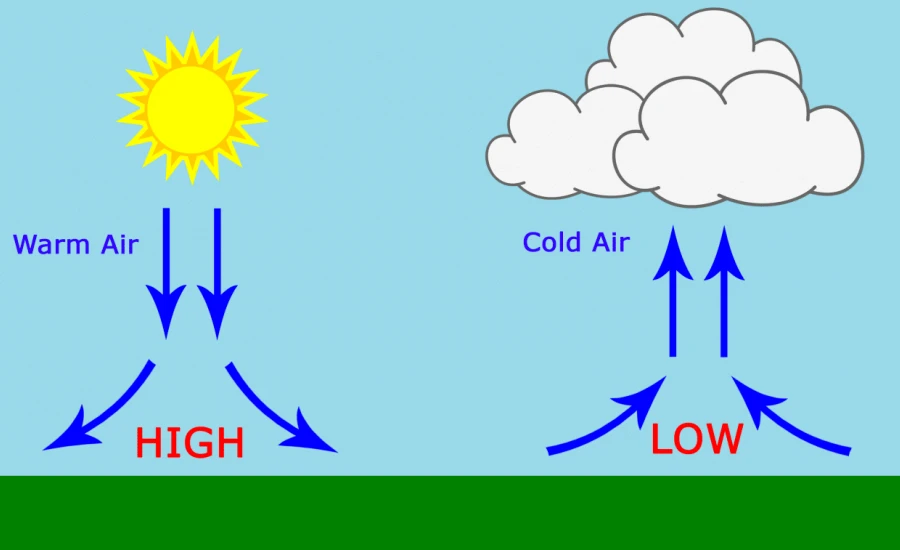
### 2. Meteorology
Atmospheric pressure variations create weather patterns. High-pressure systems typically bring clear skies, while low-pressure systems often result in cloudy, stormy conditions. Meteorologists use pressure maps to predict weather changes.
### 3. Engineering Applications
From designing aircraft that operate at varying altitudes to creating pressurized cabins for space travel, engineers must account for ambient pressure changes. Submarine designs must withstand immense water pressure at depth, while vacuum systems require containment of low-pressure environments.
### 4. Biological Systems
Living organisms adapt to their pressure environments. Human physiology, for instance, is optimized for sea-level pressure. At high altitudes or underwater, special equipment (like oxygen tanks or pressurized suits) becomes necessary to maintain proper bodily functions.
## Practical Examples of Ambient Pressure Effects
Consider these real-world scenarios where ambient pressure plays a critical role:
– Cooking: Water boils at lower temperatures at high altitudes due to reduced atmospheric pressure
– Scuba diving: Pressure increases by 1 atm for every 10 meters of depth, requiring careful decompression
– Industrial processes: Many manufacturing techniques require controlled pressure environments
– Space exploration: Spacecraft must maintain internal pressure while withstanding vacuum conditions
## Conclusion
Ambient pressure is more than just a background condition – it’s a fundamental parameter that shapes our physical world and technological capabilities. From the weather patterns above us to the biochemical processes within us, pressure variations create both challenges and opportunities across scientific fields. Understanding and measuring ambient pressure remains essential for advancing technology, predicting natural phenomena, and expanding human exploration of extreme environments.
Keyword: ambient pressure definition